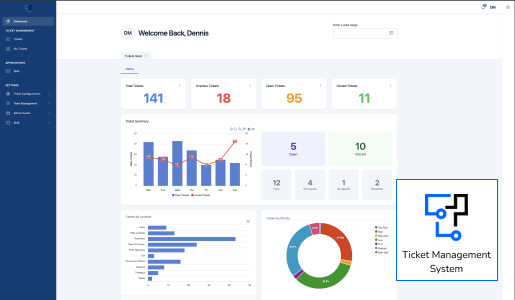
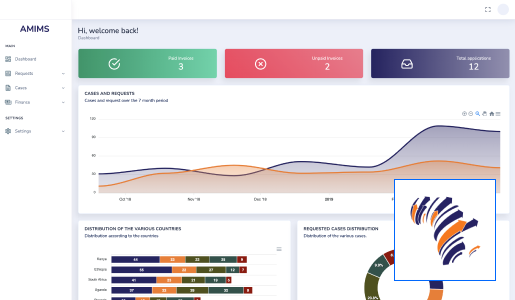
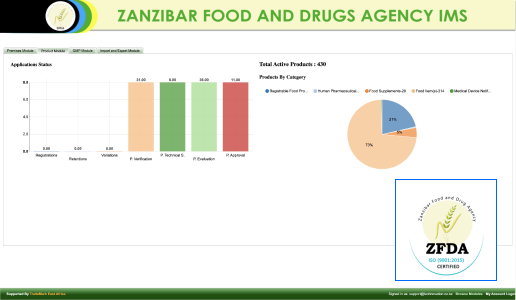
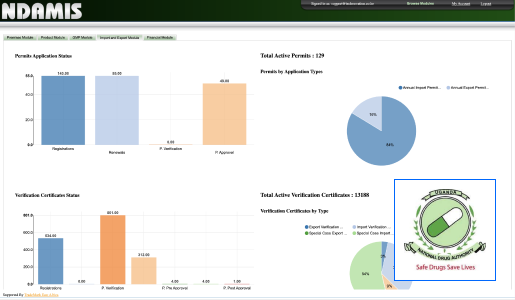
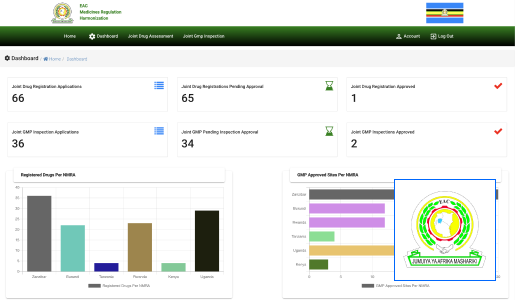
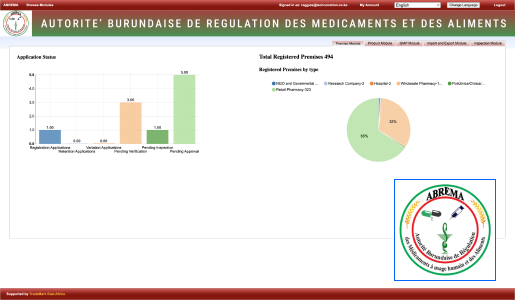
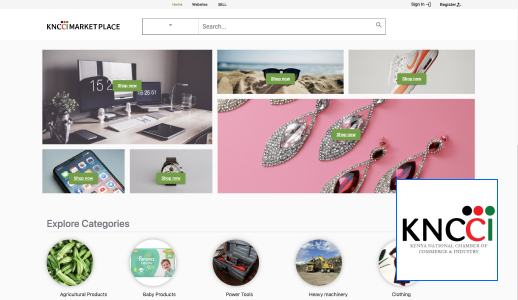
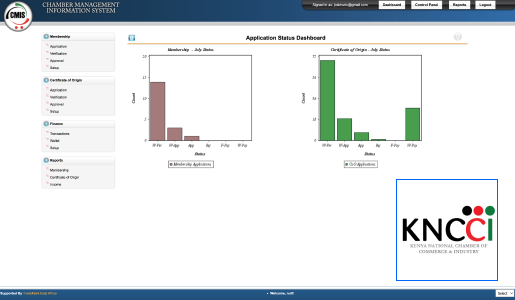
At Technovation, we are dedicated to revolutionizing the way businesses operate with our cutting-edge software engineering solutions. With a team of experienced and talented engineers, we transform your ideas into robust, scalable, and innovative software applications that propel your business to new heights.
Our Portfolio
Contact us
info@technovation.co.ke
+254 20 239 2555
+254 780 000 189
Allamano Centre (Consolata Shrine),
3rd Floor, Suite 3C,
Waiyaki Way, Westlands